Chemical Bonds: What Holds Everything Together?
Introduction
Our world that we live and breathe is filled with disorder from the moment we are born, and it is what holds everything together. Chemical bonds, to be exact, are the forces that keep molecules in place. They are by far the most critical and fundamental part of chemistry. Without them, there would be no world around us. For this article, I would like to talk about chemical bonds & the significance of them. I will briefly go over what a chemical bond is and some of its properties. Hopefully, by the end of this article, you will come to appreciate chemistry a bit more.
Why is it important to understand a chemical bond?
Chemical bonds are one of the reasons why chemical reactions take place. These “forces,” so to speak, keep molecules together and also allow others to interact with them. This scientific idea alone has helped us have a better understanding of our world. Take, for example, the first moments of life. We all start as a single cell then slowly evolve into thousands and then millions of cells, which make up our human bodies. Chemical reactions need to take place for these cells to replicate. Thus, without chemical bonds controlling these reactions, we would not be here reading these words.
Another important/significant idea to note is that without chemical bonds, there would be no oxygen for us to breathe. Millions of years ago, when our planet was inhabited, our o-zone was dangerously toxic. As time passed, the correct chemical bonds formed that gave rise to our o-zone. So not only can chemical bonds describe how we came up to be, but also explain how life was even considered to exist on earth.
Each time I think about chemical bonds, I am fascinated by just how powerful they can be. Understanding the chemical reactions of the first moments of life have reshaped how we think about our existence. Furthermore, they have allowed us to unlocked medicine, electricity, heat, and even technology! The description of what they do in our lives is limitless. As I will describe in the next section on how these differences occur.
What types of chemical bonds are there?
The types of chemical bonds can be categorized as ionic, covalent, metallic, intermolecular, and polar. Depending on the elements chosen from the periodic table, they will react differently among each other. Each has its own unique characteristics, which I will not go over in this blog post, for it will become textbook, which is something you and I do not want.
Table salt is formed by an ionic bond between sodium and chlorine. Metallic bonds are the reason electricity and heat can be transferred to us from our air conditioners in the cold winters. Polar bonds allow us to drink water after an intense day of work. Intermolecular bonds hold the nucleoside & phosphate within our DNA.
It is thus evident that chemical bonds are all around us, and trust me, they make our lives much more comfortable.
How can we describe them?
When we think of a chemical bond usually, we think of them as something physical. That is not the case, though. When physicists or chemists describe chemical bonds, they typically do it in some theoretical framework. A language basically, to give us a better way of communicating what is going on in the molecular level of our natural world. The octet rule, VSEPR theory, Valence bond theory, and Molecular orbital theory each help us grasp/describe the complexity of a chemical bond.
Here I will describe what I think is the most fascinating theory. It is called molecular orbital theory. It is what made me fall in love with physics, so there is, of course, complexity behind it.
Simply, a Molecular Orbital is a region in space in which an electron can be found in a molecule. Similar to how the ocean is a region in the world in which a shark can be found, but we don’t know where precisely one may be. These regions of space are the forces that hold the molecules together. They can be thought of as several clouds.
Here is a picture to help you visualize them.
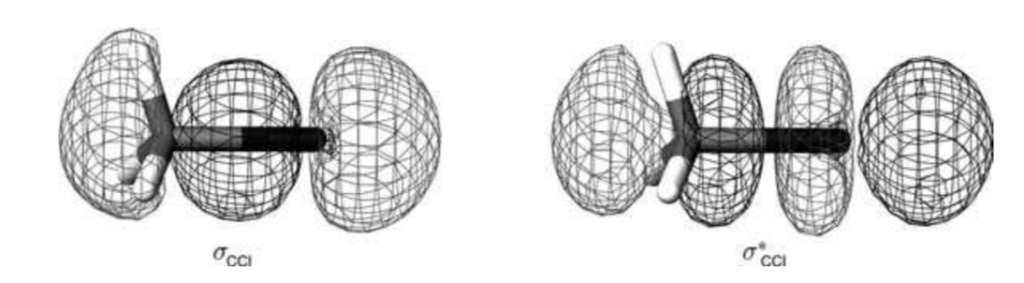
The most exciting thing is that each of these “electron clouds” can be represented by mathematics. More like a function if you may recall from elementary algebra. It looks somewhat like this!

Describing molecular orbitals and how they may behave at this framework, and I mean from chemical bonds (pure chemistry) to a function (purely mathematics), is what physicists often call Quantum Mechanics! So just by describing chemical bonds, we just merged chemistry, math, and physics. Can you see why I find them so fascinating?
Chemical bonds and Human Nature
Synthesis, Control, Static, and Never-Ending Equilibriums. This is how chemical bonds sound like. They are the foundation of what holds everything together. It is in human nature to create relational bonds with one another. Similarly, it is chemical nature to react, form, and synthesize for our world to even function. Our world continues to be changed second by second, and it is all done through the bonds that hold everything together.